58+ using a solution freezing point to calculate a molar mass
To calculate the density of water. Later by using the Kf we can measure the freezing point of the given electrolytic solution.

2 9 Determination Of The Molar Mass By Freezing Point Depression Experiment Home Version Chemistry Libretexts
Where the decrease of the frezing temperature is proportional to the number of ions the you have from the ionization of the solute i to a costant that depends also from the MM of the solvent more is big the MM more is the valour of the costant and to m that is the.

. Web Share free summaries lecture notes exam prep and more. Using a solution freezing point to calculate molar mass. Web Consider the equation for freezing point depression.
A common relation for freezing point of a solution is. M ΔTf Kfequation 1 Where ΔTf freezing point and Kf freezing constant ΔTf 544 degrees Celsius- 390 degrees Celsius 154 degrees Celsius. That is it depends on the ratio of solute and solvent particles not on the nature.
Mg of a certain molecular compound X are dissolved in 60. Using a solution freezing point to calculate molar mass - YouTube 000 534 General Chemistry 2 ALEKS. Web Choose the answer that is closest to yours.
What is the vapor pressure of a solution prepared by dissolving 1058 g naphthalene C10H8 Molar Mass 1282. This question will give you practice doing this before you come to. ΔT f i m Kf.
Calculation of the number of moles of glucoseNumber of moles of glucose Given mass of glucose Molecular mass of glucoseNumber of moles of glucose 60 g 180 g. Web O STATES OF MATTER Using a solution freezing point to calculate a molar mass When 148. 8000 g 12000 g 2000 g 10000 g What was the purpose of measuring the freezing point of water.
John 1K views 7. This property known as freezing-point depression is a colligative property. Web The number of moles of glucose Molecular weight of glucoseMass of glucose 180 gmol 60 g 0333 molThe molality of glucose solution Mass of solvent in kgNumber of.
Using a solution freezing point to calculate molar mass Roxi Hulet 58K views 1 year ago ALEKS - Calculating ideal solution composition after a distillation Tony St. Web To calculate the freezing point of an electrolytic solution we can use the same formula Tf Kf m that is used to calculate the freezing point using molality. ΔTf KfM Where m mol solutekg solvent And we know Molar mass gmol Rearranging the above equation.
Web In the Freezing Point Depression experiment you will calculate the colligative molality of the solutions you produce the freezing point depression and the predicted boiling point of the resulting solutions. Web of moles of solute added. Web The vapor pressure of carbon disulfide is 3556 torr at 25C.
But before using the formula we have to analyze the number of ions and calculate its molality.

Aleks Using A Solution Freezing Point To Calculate A Molar Mass Youtube

11 3 Colligative Properties General College Chemistry Ii

Aleks Using A Solution Freezing Point To Calculate A Molar Mass Youtube

Aleks Using A Solution Freezing Point To Calculate Molar Mass Youtube

How To Calculate Molar Mass From Freezing Point Detailed Explanation
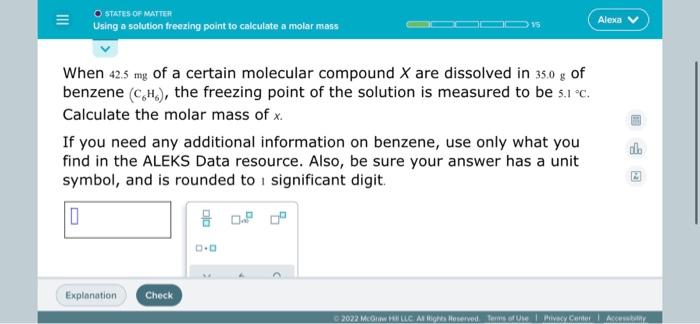
Solved O States Of Matter Using A Solution Freezing Point To Chegg Com
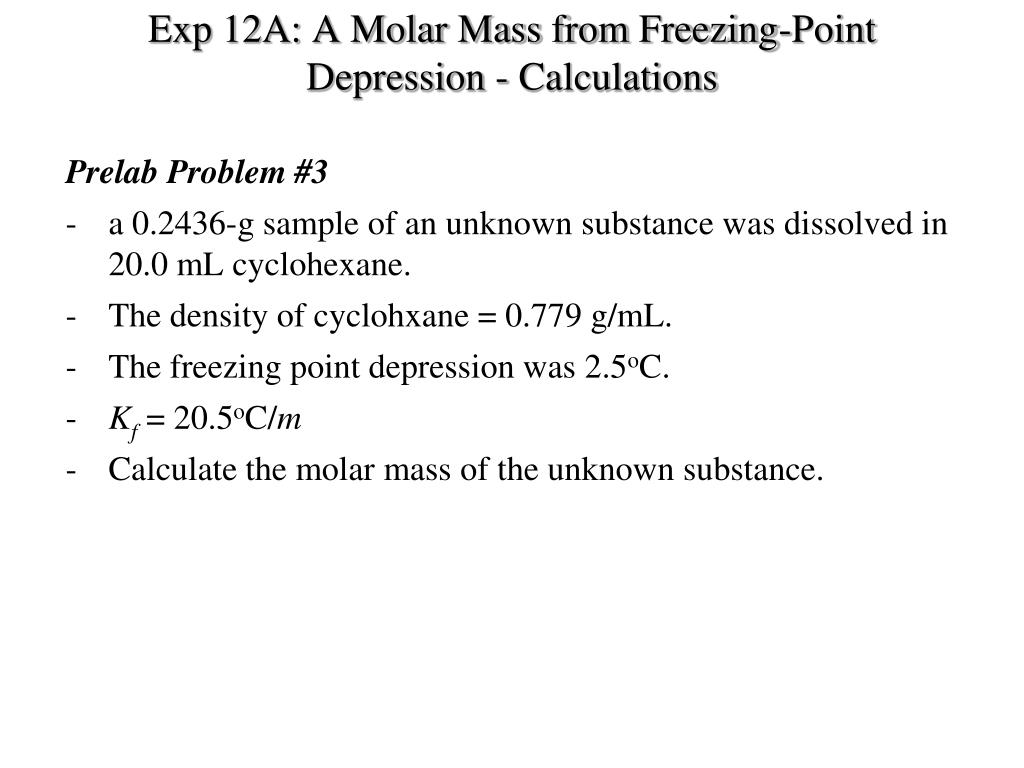
Ppt Exp 12a A Molar Mass From Freezing Point Depression Powerpoint Presentation Id 6782363

Subzero Temperature Chromatography And Top Down Mass Spectrometry For Protein Higher Order Structure Characterization Method Validation And Application To Therapeutic Antibodies Journal Of The American Chemical Society

Aleks Using A Solution Freezing Point To Calculate Molar Mass Youtube

Aleks Using A Solution Freezing Point To Calculate A Molar Mass Youtube

Aleks Using A Solution Freezing Point To Calculate A Molar Mass Youtube

Solved Determine The Freezing Point Of A Solution Which Chegg Com
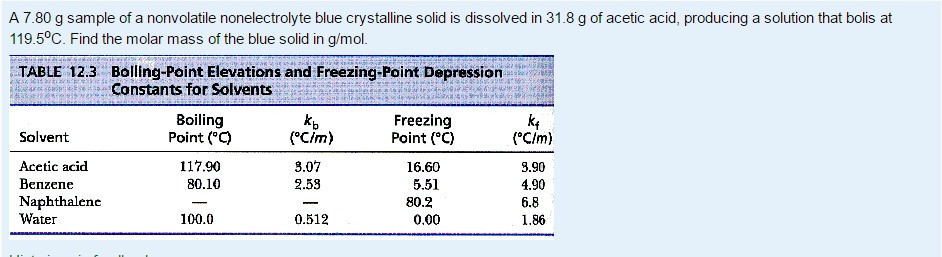
Solved A 7 80 G Sample Of A Nonvolatile Nonelectrolyte Blue Chegg Com

Aleks Using A Solution Freezing Point To Calculate A Molar Mass Youtube

Solved Using A Solution Freezing Point To Calculate A Molar Chegg Com
Class Definition For Class 516 Colloid Systems And Wetting Agents Subcombinations Thereof Processes Of Making Stabilizing Breaking Or Inhibiting

Solved The Freezing Point Of A Solvent Is 87 0 Degree C Chegg Com